Water treatment techniques that use distillation processes are among the highly efficient methods through which clean water can be obtained. The process helps eliminate a very great proportion of contaminants from the water being treated. Up to 99.9% of the contaminants in the water get eliminated through the distillation process. Despite its apparent benefits, water purification by the distillation process is not free of its challenges.
Water Distillation Explained
The water distillation process takes advantage of the principle of evaporation to separate water from contaminants. The water containing the undesirable elements is subjected to a source of heat, leading to the formation of steam. As result, large compounds fail to evaporate and do not form part of the steam. On the other hand, the evaporated steam is then subjected to lower temperatures which help it condense back to usable water.
The efficiency of using the process as the selected way of sanitizing water is therefore highly dependent on the type of contaminants in the water. For instance, many organic substances that exhibit greater boiling points tend to be effectively eliminated. A majority of these compounds might be potentially dangerous for human health when consumed. However, many other elements exhibit much lower boiling points as compared to water. As a result, if not proper mechanisms are taken to remove them, they may still b present in the eventual distillate.
Basic Principle Of Water Purification By Distillation Process
The distillation process exploits the principle of evaporation and condensation. A boiling chamber is essential with an inlet through which the unpurified water is let in. It is during this stage that heat is imparted into the mixture to allow for the formation of steam. The other main component of the system is the condensation section in which the cooling action is achieved. This receives the steam after the physical transformation of the steam into the final liquid form. Finally, some sort of storage apparatus is installed to hold the water pending consumption. Different distillation systems are available but all operate using this principle.
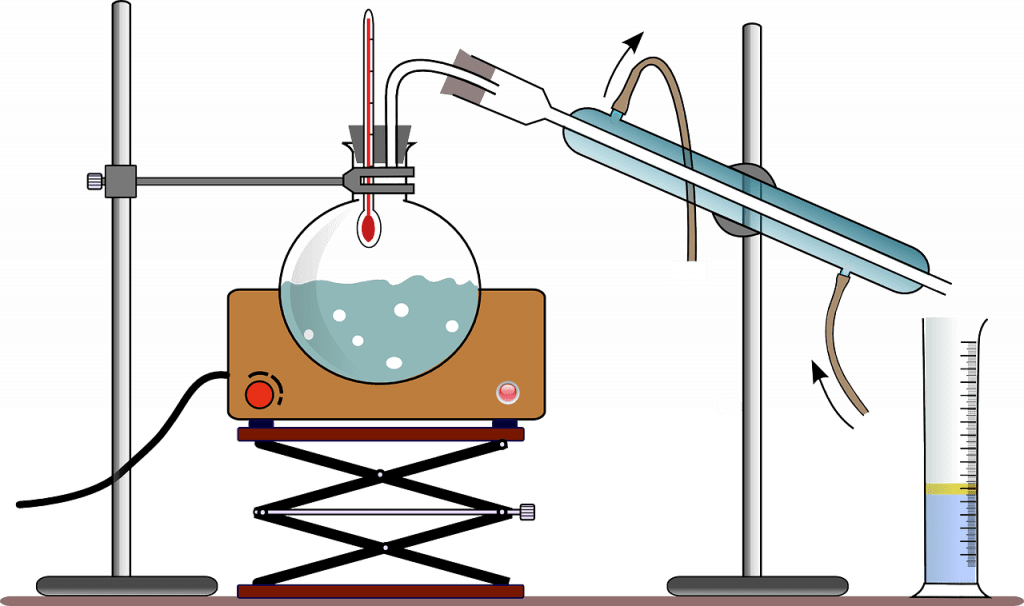
Improvements can often be made to the distillation process to ensure that high efficiencies are obtained. For example, the presence of volatile compounds in the distillate can often be a hindrance. To remove these contaminants, it is common for some setups to be installed with venting mechanisms to help remove the compounds. To achieve this, the water might initially undergo a preheating stage to temperatures below the by lining points before the main boiling stage. Water purification by boiling itself is an alternative that can be used at the domestic level.
The other improvement is in the sizes of the stills. The size ought to be based on the demand for purified water. Since distillation leads to the production of a fairly lower quantity of distillate as compared to other water purification systems and consumes more energy, their sizes must be reflective of the need.
Despite the major heating agent being electricity in most distillation setups, a variety of many other sources of fuel can be used. These include firewood and oil, which might prove to be better alternatives to electricity based on the locally available ones. Efficiencies in the process are also realized by using the inlet water to cool the steam. This imparts some of the heat energy to the incoming water, hence, reducing the amount of energy required.
Benefits Of Distillation Process
The distillation process leads to a very high elimination of microorganisms. Only a minute quantity of heat-resistant bacteria might still be present in the product. The method is one of the most efficient purification procedures available. However, the method is guaranteed to eliminate all disease-causing microorganisms that might be present.
Disadvantages Of Water Purification By Distillation Process
- Being that distillation eliminates most compounds from the water, the distillate from the process is often characterized by high corrosive potential. Hence, this increases the risk of damaging the piping materials as well as the fittings.
- The chemical compounds found in the water that are responsible for taste lack in the water. Distillation does not efficiently remove several volatile compounds as some heat-loving bacteria.
- The cost of the initial purchase of the equipment and the energy used is higher compared to other domestic water purification methods.
- Relatively small quantities of water are produced from the process.
Final Take
Since the water from distillation may easily damage the piping materials, care should be taken to ensure that only corrosion-resistant materials and fittings are used for piping. In the domestic setup, the corrosive factor may not be applicable since the distilled water may not flow through piping. The fact that only small quantities of distillate are produced means that this method might not effectively supply all the needs within a household. Hence, it should only be used to satisfy appropriate needs.
The distillation process is not efficient for the treatment of very hard water. Adsorption may be used to enhance the process. Furthermore, it is common for some compounds to scale the surface of the boiler. Hence, it is so that continuous maintenance is done to ensure that the stills operate as efficiently as possible. Otherwise, one can also obtain an automatic distillation apparatus which will minimize manual operations and maximize the operation of the setups.
Conclusion
Despite its need for specialized apparatus to achieve water purification, distillation relies on a simple principle. Furthermore, the method is so effective and produces water that is almost pure especially if the apparatus is modified to remove the volatile compounds. The principles are often used in water distillation plants for laboratory uses. Despite its success in the removal of compounds in the water, distillation might not be suitable for some instances when the local water quality is of high standards and only needs little treatment. For instance, rainwater harvested from areas without any apparent pollution does not require distillation before being consumed. However, water in dams or small lakes that receive effluents from industries might not receive the proper treatment using the conventional municipal treatment strategies. A small water distillation machine might be placed on the kitchen counter that receives some of the piped water and distills the water to be used for consumption.
